Forging the Pathway to Treatment
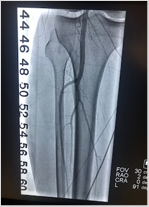
Guidewires are the most tactile-based tool you use, forging the pathway to treatment.
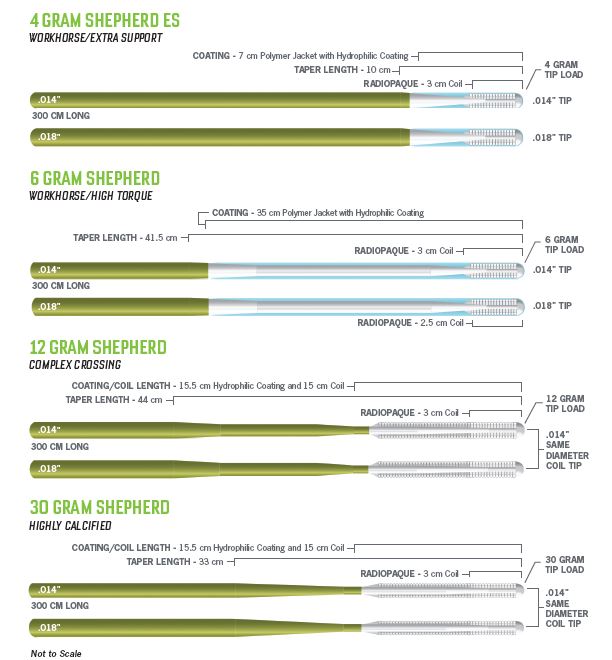
Our Shepherd Peripheral Guidewire portfolio is designed to effectively get to and cross the lesion.
Explore Shepherd Peripheral Guidewires
Hover over or click on a feature to learn more.
*When compared to predecessor Zilient® Peripheral Guidewire
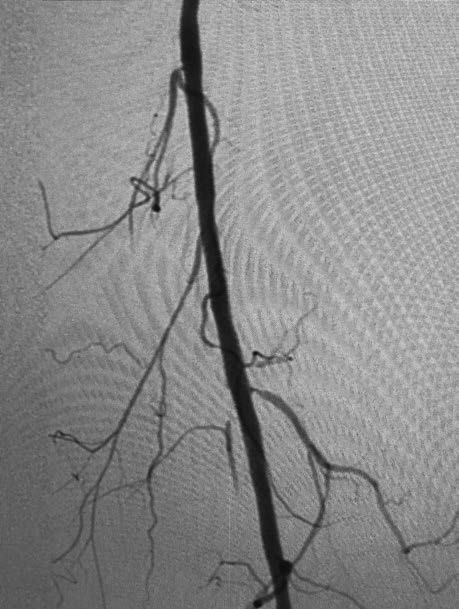
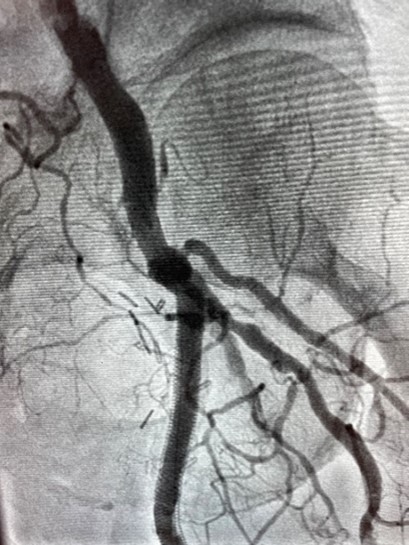
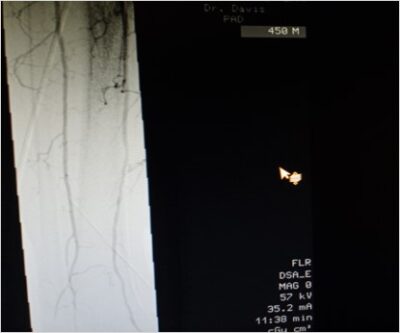
See the Shepherd Peripheral Guidewire In Action
| Model Number | Product Name | Diameter | Tip Load | Length |
|---|---|---|---|---|
| PSP14S-30004 | 014 Shepherd ES | .014" | 4 grams | 300 cm |
| PSP18S-30004 | 018 Shepherd ES | .018" | 4 grams | 300 cm |
| PSP14S-30006 | 014 Shepherd | .014" | 6 grams | 300 cm |
| PSP18S-30006 | 018 Shepherd | .018" | 6 grams | 300 cm |
| PSP14S-30012 | 014 Shepherd 12 | .014" | 12 grams | 300 cm |
| PSP18S-30012 | 018 Shepherd 12 | .018" | 12 grams | 300 cm |
| PSP14S-30030 | 014 Shepherd 30 | .014" | 30 grams | 300 cm |
| PSP18S-30030 | 018 Shepherd 30 | .018" | 30 grams | 300 cm |
More Solutions for Peripheral Artery Disease
Click the Image to Learn More
*Manufactured by OrbusNeich Medical Company Limited or its affiliates
Learn More
*Manufactured by OrbusNeich Medical Company, Limited or its affiliates
Shepherd Peripheral Guidewires
INDICATIONS: The Shepherd Peripheral guidewires are intended to facilitate the placement and exchange of balloon catheters or other interventional devices within the peripheral vasculature during Percutaneous Transluminal Angioplasty (PTA) or other intravascular interventional procedures.
CONTRAINDICATIONS: The Shepherd Peripheral guidewires are not intended for use in the coronary or cerebral vasculatures or in patients judged not acceptable for percutaneous intervention.
WARNINGS: Contents supplied STERILE using an ethylene oxide (EO) process. Do not use if barrier is damaged. For single patient use only. Do not reuse, reprocess, or resterilize. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or lead to device failure, which, in turn, may result in patient injury, illness, or death. Reuse, reprocessing, or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient. • Use the Guidewire prior to the “Use by” date on the package label, preceded by the symbol H . • Refer to the instructions supplied with any interventional devices to be used in conjunction with the guidewire for their intended uses, contraindications, and potential complications. • Do not manipulate the guidewire if resistance is met. Guidewire manipulations must always be observed under fluoroscopy. • The Peripheral family of guidewires has distal ends of varying stiffness. Operate these guidewires carefully to minimize the risk of perforation or other damage to blood vessels. • If the guidewire is removed and is to be re-inserted, it must be inspected for signs of damage (weakened or kinked segments) prior to re-introduction. Do not re-introduce if the guidewire is weakened or kinked.
PRECAUTIONS: This device should be used only by physicians trained in percutaneous, intravascular techniques and/or procedures.
Carefully read all instructions prior to use. Observe all warnings and precautions noted throughout these instructions. Failure to do so may compromise guidewire performance and result in complications. Prior to use, confirm compatibility of guidewire outer diameter with the diagnostic or therapeutic device. Inspect guidewire prior to use for any surface irregularities, bends or kinks. Damaged and/or irregular guidewires should not be used. To avoid guidewire damage, do not withdraw the wire through a metal needle cannula. The tip section of the guidewire has a proper orientation for shaping. Identify the flexing plane before shaping. Shape in the same plane as that for flexure.
Caution: Federal law (USA) restricts this device to sale by or on order of a physician.